Ben Grguric and John Toma
South Australian Museum, North Terrace, Adelaide, SA 5000
Peer reviewed (Department of State Development and externally)
Download this article as a PDF (2.1MB); cite as MESA Journal 82, pages 31–39
Introduction
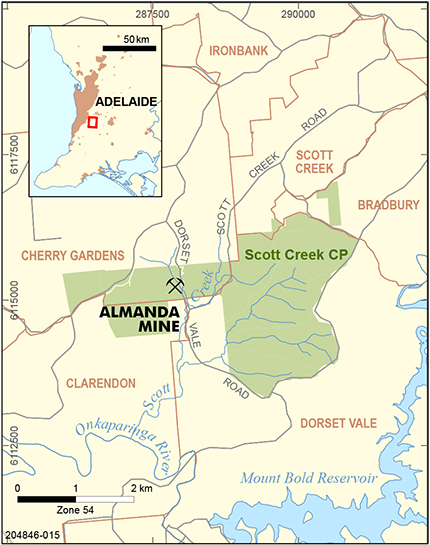
The Almanda mine, located 20 km southeast of Adelaide in the Cherry Gardens area of the Mount Lofty Ranges (Fig. 1), was a short-lived but relatively rich, 19th century silver mine. The series of shafts, shallow open cuts and tunnels exploited a single line of lode, and the Almanda mine was unusual compared to most silver deposits in the Adelaide Fold Belt in that argentiferous galena does not appear to have been the main ore mineral.
This study examines the mineralogy of historic Almanda dump material, including both oxidised and sulfide-rich samples, with the aims of determining the mineralogical hosts of silver in the ore, and understanding the genesis of the mineralisation.
Mining history
The following summary of the mining history is mainly taken from Brown (1908) and Drew (1991). The Scott Creek area including the Almanda mine was first occupied by European settlers in the 1840s. The Mackereth and Hill families settled in the area before it was officially surveyed, and set about harvesting native timber, some of which was used in construction of the city of Adelaide. Onions and potatoes were cultivated on the cleared land, and bullock-drawn wagons hauled the produce to the markets in Adelaide. In 1850 the wheel of one such wagon broke off pieces of rock revealing copper mineralisation, and mining on the site commenced. The copper mine (Wheal Maria, later renamed Wheal Mary Anne) was sunk to 30 feet on gossanous material, but was unsuccessful at an early stage of development and was soon abandoned. In 1868 a German assayer named Captain William Ey identified a significant silver content in a parcel of copper ore from Wheal Mary Anne which had been abandoned at the Port Adelaide Smelting Works. With a partner, James Gawen, he acquired the leases over the site in June 1868. A 4.5 t parcel of ore from the mine was crushed at Wentzel’s Chilean mill in Adelaide, and sufficient silver was extracted to produce two ingots of silver weighing 131 oz. These were displayed in the Adelaide Stock Exchange, and in July 1868 the Almanda Silver Mining Company was formed. The publicity associated with the silver discovery resulted in a rush in the Scott Creek area, with a total of 235 claims being pegged. Only the Almanda and Potosi claims were worked to any extent, however.

Figure 2 Sketch of the Almanda mine area and surface plant in 1868 by WA Cawthorne. (Courtesy of Mitchell Library, State Library of New South Wales)
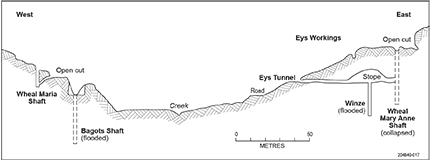
By August 1868, 20 men were working the Almanda site under the supervision of Captains Henkel and Ey, constructing a 10-head stamping battery and developing mine workings on both sides of the creek valley (Fig. 2). The site of the original copper discovery included the original Wheal Maria shaft and open cut together with the nearby Bagots Shaft, while on the western side of the valley an open cut, vertical shaft and tunnel with a winze (Eys Tunnel) on the line of lode were collectively referred to as Eys Workings (Fig. 3). Processing of the ore was performed by crushing with a 10- and later 15-head stamp battery, followed by amalgamation with mercury in a Chilean mill (later amalgamation pans), separation of the amalgam, and retorting of the latter in a furnace to produce unrefined silver ingots. In 1870 fresh assays from various parts of the mine workings yielded 57 to 66 oz of silver per ton (1,154 to 2,020 ppm Ag) and ~ 5% Cu (Brown 1908). Despite the high silver grades, the operation proved uneconomic; production was suspended in 1870 and the company dissolved in 1871, with the machinery, buildings and plant being sold for £650. Reworking of the mine occurred again in 1877, 1881 and 1887 and total life-of-mine production is estimated at 10,000 oz (310 kg) of silver from 2,000 t of ore. The Inspector of Mines reported in November 1900 that the underground workings were in a poor state of repair; however, samples from the ore dumps assayed 2,189 ppm Ag and 1.75% Cu (Brown 1908) testifying to the former richness of parts of the lode.
Between 1968 and 1972, the South Australia Department of Mines re-evaluated the Almanda mineralisation and undertook induced polarisation geophysical and stream and soil geochemical sediment surveys in the mine area (Binks 1968; Rowan 1969), followed by four diamond drillholes (DAL1 to DAL4) along the line of lode for a total of 438 m. Two of the drillholes failed to intersect the mineralised lode: DAL1 encountered anomalous Cu, Pb, Zn and Ag; and DAL2, drilled under Eys Workings, intersected a low-grade ore shoot between 70 and 78 m downhole with maximum assays of 0.81% Cu, 184 ppm Ag, 2,950 ppm Pb, 6,750 ppm Zn and 1.1% As (Mosig and Fidler 1972). The final assessment of the drilling program was that there was an estimated 6,000 t of remaining mineralisation, but low grades and the narrow true-width (1 m) of the lode indicated that it would be uneconomic to mine. It was inferred that any further undiscovered ore reserves would probably be of similar magnitude and grade.
Geology

Figure 4 Current site of Bagots Shaft overlooking the creek valley. (Photo 415145)

Figure 5 View along the line of lode, looking east, Wheal Mary Anne open cut, indicating the approximate width and steep dip of the mined-out lode. (Photo 415146)
The various workings at the Almanda mine (Figs 4, 5) exploited a single line of lode hosted within shallowly dipping phyllites of the Saddleworth Formation, which at depth is underlain by the Stonyfell Quartzite. Both of these country rock units are Late Torrensian in age (c. 800 Ma) and the package lies between the Willunga and Ochre Cove faults. The mineralised lode was E–W-striking, 0.9–1.5 m wide and 850 m in strike length, with a steep 80–90° dip to the north. Rowan (1969) noted that contacts with the wall-rock were sharp, and that the accessible lode exposed in Eys Tunnel consisted of subparallel veins separated by altered phyllite for a total lode width of 0.9–1.5 m. Given the obvious structural control of the mineralised lode (fault or shear-hosted), its orientation and planar morphology, it is probable the Almanda primary mineralisation was deposited by hydrothermal fluids during the Delamerian Orogeny (514–500 Ma; Foden et al. 2006).
Near-surface lode material is dominated by dark-brown, gossanous material and lesser fragmental vein quartz. Within this lode high-grade mineralisation was apparently concentrated in shoots pitching –50° to –60° to the east (Hughes 1953). These were worked to a vertical depth of 91 m. Brown (1908) noted that below 30 m the lode ‘assumed a hard character and contained barytes, quartz, silver ore, arsenic &c’. Where fractured Stonyfell Quartzite was intersected at depth, e.g. in drillhole DAL1, a high flow rate of groundwater was recorded (7,500–11,350 L/h; Rowan 1969), and Brown (1908) noted that when the winze in Eys Tunnel was sunk to 139 m in 1881 ‘the water came in too strongly to be kept under by hand labour’. It is therefore assumed that poorer grades and higher pumping costs associated with mining at increasing depths were instrumental in the mine closing.
Analytical methods
At the initiation of the study, it was found there were no existing specimens from the Almanda mine in the South Australian Museum mineral collection, a surprising fact considering the collection incorporates the former South Australia Mines Department collection rich in representative ores from historic South Australian mines. One of us (J Toma) has been a lifetime mineral collector in South Australia, and had obtained samples from the mine dumps prior to the mine area being incorporated into the Scott Creek Conservation Park in 1985. This suite of samples was donated to the South Australian Museum collection, and forms the basis for the present study.
Dump specimens consisted of both sulfides in a carbonate–quartz gangue and gossanous material. Most of the sulfidic material showed evidence of incipient surface to partial oxidation due to exposure in the dumps for over 100 years, however, larger specimens were remarkably fresh internally. This is presumably due to their high carbonate gangue content. Specimens were cut on a 6 inch diamond saw and mounted in 25 mm round mounts using cold-set epoxy resin. These were ground on a diamond lap and polished on cloth laps using monocrystalline diamond pastes.
The polished sections were examined using reflected light optical microscopy with an Olympus BH2 microscope equipped with a digital camera. Scanning electron microscopy and energy dispersive (EDS) microanalysis was carried out on a Philips XL30 scanning electron microscope (SEM) equipped with an EDS detector at Adelaide Microscopy. Samples were carbon coated for SEM examination. Powder X-ray diffraction analyses were performed on the samples using a Huber 670 Diffractometer equipped with an image plate Guinier camera.
Results
Sulfide-rich samples
The only previous petrographic examination of Almanda sulfide-bearing mineralisation known to us appears in Binks (1968, mineragraphic description MP2210/68). This report refers to a single sample of dump material consisting of arsenopyrite (FeAsS) and small blebs and massive areas of chalcopyrite (CuFeS2) with inclusions of pyrite (FeS2), tetrahedrite ((Cu,Fe,Zn,Ag)12Sb4S13) and native bismuth in a gangue of crystalline dolomite, siderite and quartz. As part of the present study a more comprehensive suite of 25 sulfide-bearing dump samples was examined. Very little primary sulfide was found in samples collected from Eys Tunnel dumps aside from some relict arsenopyrite and chalcopyrite in gossan; however, sulfide-rich material was relatively abundant in dumps in the vicinity of the Wheal Maria Open cut and Shaft and Bagots Shaft.
Fresh samples were of two basic types:
- Type 1. Brecciated vein quartz containing abundant disseminated pyrite (Fig. 6a).
- Type 2. Brecciated and recemented grey-blue to fawn-brown siderite containing abundant disseminated to microcrystalline arsenopyrite and chalcopyrite (± pyrite). Brecciated fragments of vein quartz were locally present (Fig. 6b). In the dump material examined, this carbonate-rich type was by far the most abundant.
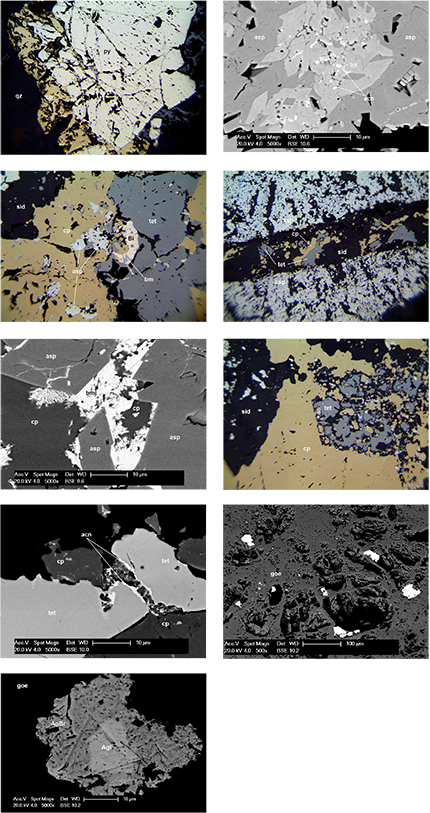
Optical and SEM microscopy of the Type 1 sulfidic samples showed them to consist of 0.1–1 mm, rounded to subhedral pyrite crystals, most of which were microfractured and the fractures infilled with chalcopyrite in a matrix of crystalline vein quartz (Fig. 7a). Chalcopyrite also filled interstices between clustered pyrite crystals. Minor arsenopyrite crystals to 100 µm were noted, and traces of galena (PbS) occurred in the form of <10 µm inclusions in both pyrite crystals and quartz. No obvious compositional zoning was detected in pyrite crystals using EDS microanalysis.
Type 2 sulfidic samples consisted predominantly of arsenopyrite in a gangue of microcrystalline siderite. The modal proportion of arsenopyrite varied from fragments of essentially massive, crystalline sulfide with minor inclusions of siderite to abundant disseminations of 10–100 µm, euhedral arsenopyrite crystals in siderite. A common texture was unusual 100–200 µm lath-like aggregates of crystalline siderite within a matrix of finer grained, polycrystalline arsenopyrite. Minor anhedral quartz was also present. In SEM backscattered electron mode, most crystals of Almanda arsenopyrite were seen to contain inner cores of earlier formed löllingite (FeAs2), and locally, minute inclusions of acanthite (Ag2S) were noted in both löllingite and the arsenopyrite overgrowths/replacements (Fig. 7b).
Chalcopyrite is relatively abundant in Type 2 samples in the form of anhedral bodies to several millimetres across in siderite. Closely associated sulfides were anhedral to subhedral crystals of tetrahedrite to 300 µm, and patches and inclusions of native bismuth, variably replaced by bismuthinite (Bi2S3; Fig. 7c). In some samples, pyrite crystals to 150 µm occurred as inclusions in chalcopyrite, however, the pyrite association was not common. The Cu–Bi-rich association is clearly paragenetically later than the arsenopyrite–löllingite stage and inclusions of arsenopyrite within chalcopyrite locally showed caries textures indicative of partial replacement. Examples were also observed of veinlets of siderite containing chalcopyrite and tetrahedrite crosscutting earlier formed arsenopyrite bodies (Fig. 7d). Native bismuth was also locally observed interstitial to arsenopyrite, and locally bismuthinite was observed replacing chalcopyrite (Fig. 7e). The brecciated and recemented nature of siderite-rich samples suggests multiple hydrothermal pulses, and consequently the paragenesis of this material is not straightforward. In places tetrahedrite appears in apparent textural equilibrium with chalcopyrite, while in others chalcopyrite appears to replace tetrahedrite. For example, sample Figure 7f shows a former euhedral crystal of tetrahedrite now partially replaced by mixture of chalcopyrite, siderite, native bismuth, minor sphalerite (ZnS) and a few grains of a Ag–Bi–S phase, which shows relatively strong optical anisotropy, likely a member of the pavonite homologous series, possible pavonite ((Ag,Cu)(Bi,Pb)3S5) or benjaminite ((Ag,Cu)3(Bi,Pb)7S12). Tetrahedrite was also observed with very thin replacement rims (<2 µm thick) of acanthite (Fig. 7g). Microanalysis of the sulfide-rich samples showed that the relatively abundant tetrahedrite contained between 12.9 and 21.4 wt% Ag in solid solution. The tetrahedrite was arsenic-poor (<3 wt%) and also contained between 2.8 and 4.3 wt% Zn. No mercury or tellurium was detected in Almanda tetrahedrite using EDS analysis, the detection limit of which is in order of 0.5 wt%.
The chemistry of the siderite gangue, as determined by EDS microanalysis, varied considerably and non-systematically between early brecciated fragments, breccia matrix and late, crosscutting veins, with variation defined by Mg (2.2–49.6 at.%) and Mn (1.7–15.0 at.%) substituting for Fe. No dolomite was detected in the Almanda samples analysed here, and Ca substitution in siderite was in all examples <2.5 at.%. Importantly, barite was not observed as a significant gangue phase in any of the sulfidic samples. We suggest that the earlier erroneous identification of barite (e.g. in Brown 1908 and repeated in later references) may have been a result of the fact that fresh Almanda siderite is often blue-grey in colour and commonly heavily included with arsenopyrite, resulting in a high specific gravity of hand specimens of the ore. Confirmed barite of a similar colour and crystalline texture is abundant at the nearby Mount Malvern Pb–Ag mine, adding to the confusion. In this study, the only barite found in Almanda samples was a trace amount in a single gossan sample from the Eys Tunnel dumps. The other notable rarity was galena, a mineral which is common in Pb–Ag ores from the Adelaide Fold Belt and typically forms the main host of silver in these systems. As mentioned, galena was identified in Type 1 sulfide samples but only in trace amounts.
Gossanous samples
Nine gossanous dump samples were examined as part of the present study. These were collected from both the Wheal Maria and Bagots shaft dumps and the Eys Tunnel dump. The gossans consisted predominantly of goethite and other iron-oxide/hydroxides together with relict vein quartz and, locally, trace to minor relict arsenopyrite grains. Relict textures in gossans included boxworks after sulfides, and more commonly rhombic microtextures after siderite.
In SEM backscattered electron mode, rounded blebs and small crystals of silver halides were readily recognised in Almanda gossan samples due to their brightness against the iron-rich gossan matrix. The most common mode of occurrence of the halides was as blebs 2–60 µm across infilling pores and attached to the hollow walls of goethite boxworks (Fig. 7h). Silver halides were only identified in Eys Tunnel dump samples, possibly because they represented the remnant run-of-mine ore left when mining at Almanda ceased. EDS microanalysis showed the silver halides to consist of two species: iodargyrite (AgI) with up to 17 at.% Br substitution; and chlorian bromargyrite (Ag(Br,Cl)) with 35–45 at.% Cl substitution. Within larger grains, clear compositional zonation was detected in backscattered mode, the cores being iodargyrite and the rims being chlorian bromargyrite (Fig. 7i). No other silver minerals such as native silver or ‘ruby silver’ (i.e. pyrargyrite, proustite) were identified, suggesting the silver halides observed were the main host of silver in the oxide zone ores. Chlorargyrite (AgCl) from Almanda was listed in Brown (1908) but was not identified in this study.
Discussion
Silver mineralogy and implications for ore processing
Although minor acanthite was noted in some samples, silver-bearing tetrahedrite was always present in far higher modal quantities and thus appears to be the main host of silver in primary zone, sulfide-rich samples from the Almanda mine.
Based on our analysis of gossanous samples, silver halides, namely bromargyrite and iodargyrite, would appear to have been the main host of silver in the oxide zone at Almanda. Little detail is available regarding the exact process used to extract silver from Almanda ores other than it involved amalgamation with mercury. Conventional crushing and grinding of the oxide ore containing silver halides in the presence of liquid mercury would readily result in the formation of silver-bearing amalgam, and this process would also recover any free gold, native silver or electrum present in oxide zone ore as well. Simple retorting of the separated amalgam would yield unrefined silver (+ gold) which could be melted into bars.
In the case of the sulfide-rich ores encountered at depth at Almanda and now present on the dumps, silver recovery using simple amalgamation would not seem likely to be effective, and poor metal recoveries would be expected. A modification of the amalgamation process used extensively in South and Central America to treat silver sulfide ores from the 1550s onwards was the so-called Patio process (Waszkis 1993). Finely ground acanthite-bearing ore was mixed with water, salt, copper sulfate and mercury and manually or mechanically kneaded for up to 30 days. This resulted in acanthite being converted to chlorargyrite (AgCl) which could then combine with the mercury to form silver amalgam. How effective such a process would be in recovering silver from tetrahedrite is unknown, and it has not been established if the Patio process, a related method (e.g. the pan or Washoe process), or roasting of sulfides prior to amalgamation was utilised at Almanda. Early accounts mention the recovery of ‘dirty amalgam’ during treatment of Almanda ores (Wells 1976), which may well have been a consequence of attempts to amalgamate sulfide ores containing the native bismuth observed in polished section. Binks (1968) surmised that the sulfide ore, being fine grained and disseminated through hard, crystalline dolomite gangue, would have made crushing and ore dressing difficult. We suggest the combination of hard ore, the rudimentary processing and basic metallurgical options available at the time, the lower grade of the hypogene sulfide mineralisation, and water influx at depth were instrumental in ending mining activities at Almanda in the 19th century.
Genesis of the Almanda mineralisation
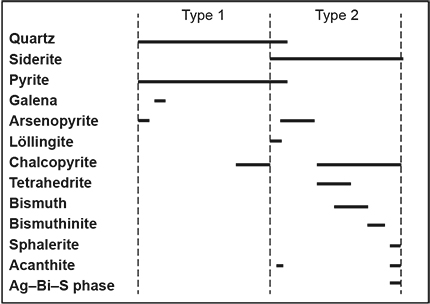
Figure 8 Proposed paragenetic sequence for primary ore from the Almanda mine based on samples examined in this study.
A proposed paragenetic sequence for the primary zone mineralisation at Almanda is presented in Figure 8, based on petrographic observations. Type 1 mineralisation dominated by vein quartz and pyrite, is followed by deposition of siderite gangue with arsenopyrite and later chalcopyrite, tetrahedrite and bismuth phases in Type 2 mineralisation. Localised replacement of tetrahedrite by chalcopyrite and siderite resulted in the formation of minor sphalerite, acanthite and an unidentified Ag–Bi–S phase. The sequence of quartz followed by siderite as the dominant gangue phase suggests infiltration of a silica-rich, CO2-poor fluid followed by a more CO2-enriched fluid. Quartz is relatively insoluble in CO2; however, precipitation of siderite in the second stage would remove CO2 from the fluid which would increase the solubility of silica, thus inhibiting further quartz precipitation and possibly redissolving existing Type 1 quartz (e.g. see Fougerouse et al. 2016). Such a process might explain why Type 1 material was rare on the dumps relative to Type 2 carbonate-rich material, and why vein quartz was generally found in early formed fragments in siderite-rich breccia (e.g. Fig. 6b). As mentioned, the clear structural control and morphology of the lode, as well as the style and metal association of the mineralisation would suggest the Almanda ores were hydrothermal (mesothermal) in origin and are postulated to have deposited relatively late in the Delamerian deformation episode. The hydrothermal fluids may have been metamorphic in origin or derived from an unmapped igneous body at depth.
The oxide zone ores at Almanda appear to have been dominated by secondary goethite derived from oxidation of both iron-bearing sulfide minerals and siderite gangue. The presence of the insoluble silver halides in the oxide zone above silver-bearing sulfide systems is to be expected, and is particularly characteristic of oxide zones developed in arid or semi-arid climates (Guilbert and Park 1986). The observed replacement of iodargyrite by chlorian bromargyrite (Fig. 7i) may reflected the initial formation of the least soluble halide (AgI) followed by replacement by bromine- and chlorine-bearing species under progressively higher states of oxidation (Sillitoe 2009). The chlorine, iodine and bromine in these minerals may be derived from windborne salts (e.g. from seawater) or, alternatively, from fluid inclusions in the ore released when the host minerals were decomposed during weathering.
Conclusion
Silver in Almanda mine ores was hosted in silver-bearing tetrahedrite and minor acanthite in primary ores, and silver halides (bromargyrite and iodargyrite) in the oxide zone. Unlike most silver mines in the Adelaide Fold Belt, galena appears to have been uncommon. Although supergene-enriched sulfide ores may have been present, no definitive examples were identified in the suite of dump samples examined. Silver-rich oxidised zones are, however, commonly developed in many outcropping, partially oxidised deposits where the silver enrichment is either of residual or chemical origin (Sillitoe 2009). We surmise that the short economic mine life of the Almanda workings was due to the limited tonnages of easily crushed and amalgamated oxide zone ore, which gave way to harder and more less-readily processed mineralisation at depth, compounded by high groundwater influxes once the lode passed into fractured Stonyfell Quartzite country rock.
Acknowledgements
Ben Nicolson (Geological Survey of South Australia) and Nigel Cook (University of Adelaide) are thanked for their reviews of this article.
References
Brown HYL 1908. Record of the mines of South Australia. 4th edn. Government Printer, Adelaide.
Drew GJ 1991. Almanda silver mine: a guide to the walking trail. South Australia Department of Mines and Energy, Adelaide.
Foden J, Elburg MA, Smith PB, Dougherty-Page J and Burtt A 2006. The timing and duration of the Delamerian orogeny: correlation with the Ross Orogen and implications for Gondwana assembly. The Journal of Geology 114:189–210.
Fougerouse D, Micklethwaite S, Tomkins AG, Mei Y, Kilburn M, Guagliardo P, Fisher LA, Halfpenny A, Gee M, Paterson D and Howard DL 2016. Gold remobilisation and formation of high grade ore shoots driven by dissolution-reprecipitation replacement and Ni substitution into auriferous arsenopyrite. Geochimica et Cosmochimica Acta 178:143–159.
Guilbert JM and Park CF 1986. The geology of ore deposits. WH Freeman and Co., New York.
Sillitoe RH 2009. Supergene silver reassessed. In SR Titley ed., Supergene environments, processes and products, Special Publication 14. Society of Economic Geologists, pp. 15–32.
Waszkis H 1993. Mining in the Americas: stories and history. Woodhead Publishing Limited, Cambridge.
Wells R 1976. A history of the mines in the Cherry Gardens to Kangarilla area. Unpublished report.